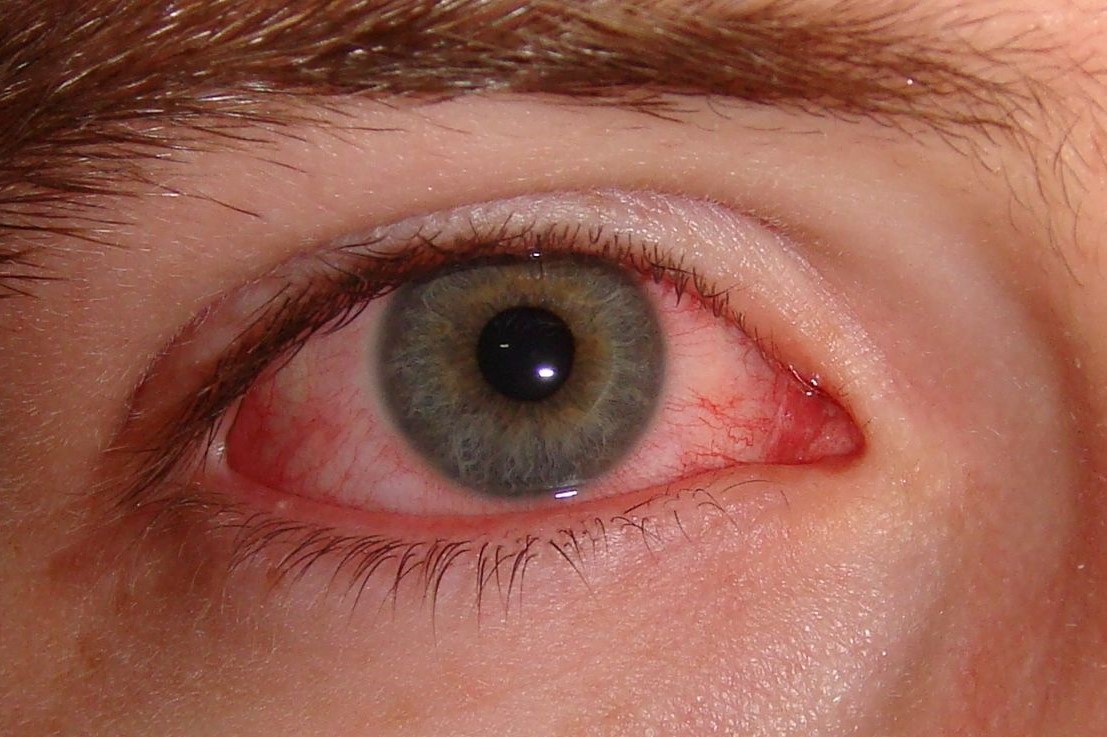
Promising conjunctivitis treatment
Aldeyra Therapeutics has initiated the phase III trial to assess the efficacy and safety of topical reproxalap ophthalmic solution (0.25%) as a treatment for allergic conjunctivitis.
“Initiating enrolment in the phase III trial moves us closer to our goal of providing allergic conjunctivitis patients with a new treatment option for one of the world’s most common ocular conditions,” said Aldeyra’s president and CEO Dr Todd Brady.
The randomised, double-masked, crossover vehicle-controlled phase III will enrol approximately 120 patients and, consistent with prior allergic conjunctivitis trials, the primary end point will be a subject-reported ocular itching score.
In Aldeyra’s phase II trial, completed in March 2019, 0.25% reproxalap demonstrated significant reductions in ocular itching and redness.