The role of inflammation in dry eye
Inflammation has been implicated in the pathogenesis of dry eye disease (DED)1,2, most notably in conditions such as Sjögren’s syndrome. However, in meibomian gland disease, considered the most common cause of dry eye, a lack of evidence confirming inflammation within the glands, suggests that ocular surface inflammation in this condition exists as a sequela of ocular surface insult. Indeed, in the context of the self-perpetuating nature of dry eye disease, it is not inconceivable that both initiating and downstream inflammation can occur³-⁵.
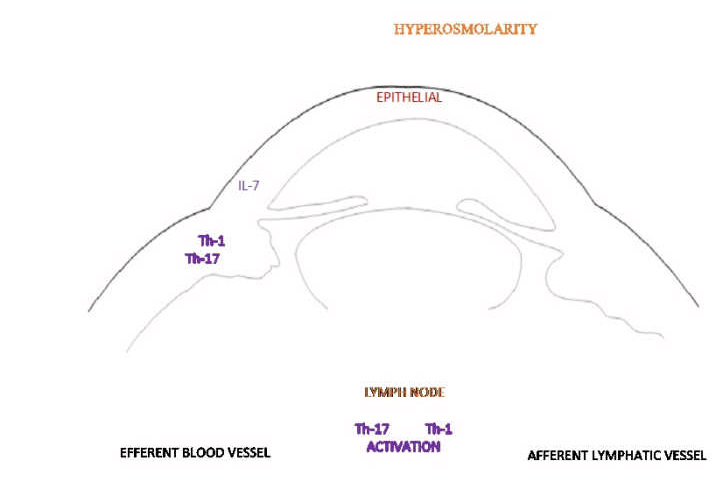
Hyperosmolarity is a fundamental feature in DED, irrespective of the dry eye subtype⁴ and is the likely trigger of the acute immune response³. Stress induced by desiccation provokes epithelial activation stimulating the production of inflammatory mediators at the ocular surface⁵,⁶. The subsequent activation of an adaptive immune response drives the inflammatory cascade⁴. As shown in Fig 1, tear hyperosmolarity specifically activates intracellular signalling pathways causing the production of proinflammatory cytokines (interleukin-1, interleukin-6 and tumour necrosis factor-alpha). This activates mature antigen-presenting cells which migrate via the afferent lymphatic system to the draining lymph node. Here they induce effector T cells, namely T helper-1 and T helper-17, which migrate via the efferent vasculature to the ocular surface. Interferon-gamma and interleukin-17 are secreted by the effector T cells which further promote pathogenic effects via the production of proinflammatory cytokines, chemokines, matrix metalloproteinases and cell adhesion molecules, leading to damage at the ocular surface. Causing injury to the corneal and conjunctival epithelial cells prompts changes that include cell death by apoptosis, goblet cell loss and reduced mucus secretion³ and contributes to further tear film instability and increased hyperosmolarity, thereby perpetuating the ‘vicious cycle’³.
Epitheliopathy, neuropathy and lymphangiogenesis are three immune-mediated changes that occur in DED. Clinically, epitheliopathy is the most widely recognised feature of ocular surface disease, in part due to the ease and practicality of administering diagnostic surface stains such as sodium fluorescein and lissamine green. The matrix metalloproteinases, that are produced in response to desiccating stress, promote corneal extracellular matrix destruction and epithelial cell loss⁶. Interleukin-17 has also been shown to disrupt corneal epithelial barrier function⁷.
Abnormalities in corneal nerve morphology have been shown to correlate with DED severity⁸. A healthy cornea has nerve endings protected between layers of epithelial wing cells, however, the inflammatory-mediated epitheliopathy results in exposure of the nerves to mechanical and inflammatory insult. Inflammatory cytokine exposure increases the synthesis of neurotrophic factors that stimulate nerve growth, often with reduced sensitivity and altered sub-basal nerve morphology.
The creation of afferent lymphatic vessels and blood vessels is usually the result of a coordinated mechanism. However, in DED, lymphangiogenesis occurs without associated haemangiogenesis⁹. Lymphangiogenesis is promoted by the expression of vascular endothelial growth factors and associated receptors. These newly formed lymphatic vessels in the cornea are a means by which antigens and antigen-presenting cells travel between the ocular surface and lymph nodes. Hence the inhibition of lymphangiogenesis could also be a potential therapeutic target for managing DED¹⁰.
As described, ocular surface inflammation is propagated by the activation of an acute and subsequently an adaptive immune response. This cascade incites dysregulation of the immune system. Symptoms associated with these events include burning, irritation, redness, photophobia and blurred vision. The chronic nature of the disease process can cause permanent alterations to the ocular surface and adnexa. Arresting disease progression to manage symptoms and signs is therefore a critical part of the eyecare professional’s role.
An increasing investment in treatment options that target the source of dysfunction can break the vicious cycle and help manage the resulting inflammation. Where there is a need to tackle ocular surface inflammation head-on, positive outcomes may be achievable with novel steroid-sparing immunomodulatory agents such as cyclosporine A (Restasis) and lifitegrast (Xiidra), that have received regulatory approval in the US. Until such time as these, or equivalent, commercial products, are approved for supply in New Zealand, access remains restricted to generic products created by compounding pharmacies (for cyclosporin A) or to costly import of commercial variants by a medical practitioner via section 29 of the Medicines Act.
References
- Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens 2014; 40: 248–256.
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 2012; 130: 90–100.
- Bron AJ, de Paiva CS, Chauhan SK et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017; 15: 438–510.
- Baudouin C, Messmer EM, Aragona P. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol 2016; 100: 300–306.
- Pflugfelder SC, de Paiva CS, Li DQ et al. Epithelial immune cell interaction in dry eye. Cornea 2008; 27: S9–S11.
- Corrales RM, Stern ME, De Paiva CS et al. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci 2006; 47: 3293–3302.
- De Paiva CS, Chotikavanich S, Pangelinan SB et al. IL- 17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2009; 2: 243–253.
- Lee HK, Ryu IH, Seo KY et al. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 2006; 113: 198–205.
- Goyal S, Chauhan SK, El Annan J et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol 2010; 128: 819–824.
- Skobe M, Dana R. Blocking the path of lymphatic vessels. Nat Med 2009; 15: 993–994.
Optometrist and researcher Varny Ganesalingam holds a Master of Health Sciences in Ophthalmology from the University of Auckland and is currently a staff optometrist with the Australian College of Optometry.