Contemporary approaches to assessing and treating diabetic eye disease
Diabetes has been described as one of the biggest epidemics of the 21st century. By 2045, 783 million people are projected to have developed diabetes globally1. In 2021 alone, diabetes resulted in 6.7 million deaths worldwide and US$966 billion in global healthcare spending1.
The effect of diabetes on ocular health is evident, with an estimated global incidence of patients affected by diabetic macula oedema (DMO) approaching 20.6 million, and 17.2 million affected by proliferative diabetic retinopathy (DR)2. With the rising prevalence of diabetes both in developed and developing countries comes an increased risk of DR and potential vision loss. Effective, timely diagnosis and treatment of DR is vital to prevent vision loss. Thankfully, progress has been made in the identification and management of diabetes-related eye disease. In countries such as the UK, the utilisation of nationwide DR screening programmes has resulted in lower rates of vision loss3 and has been credited for diabetes no longer being the leading cause of blindness in the working-age population in England and Wales4.
Clinical assessment of DR
Colour fundus photography is the standard imaging modality in many retinal screening programmes. However, this visualises only a third of the retina per fundus image5, requiring multiple images to be captured per patient.
Newer technology such as widefield fundus photography now allows us to capture more than 80% of the retina in a single photo6. Evidence is emerging that peripheral retinal findings not previously seen on standard fundus photographs have an important role in assessing risk for retinopathy progression7. Consequently, there have been calls from the medical retina community for a new staging system for grading diabetic retinopathy which includes widefield imaging features8.
Optical coherence tomography (OCT) has become a mainstay investigation when assessing the macula for diabetic maculopathy. This technology allows clinicians to non-invasively image retinal structures and evaluate them either en-face or in cross section for morphological changes that may affect visual function. Serial OCTs also provide an excellent method of monitoring disease progression over time and response to treatment.
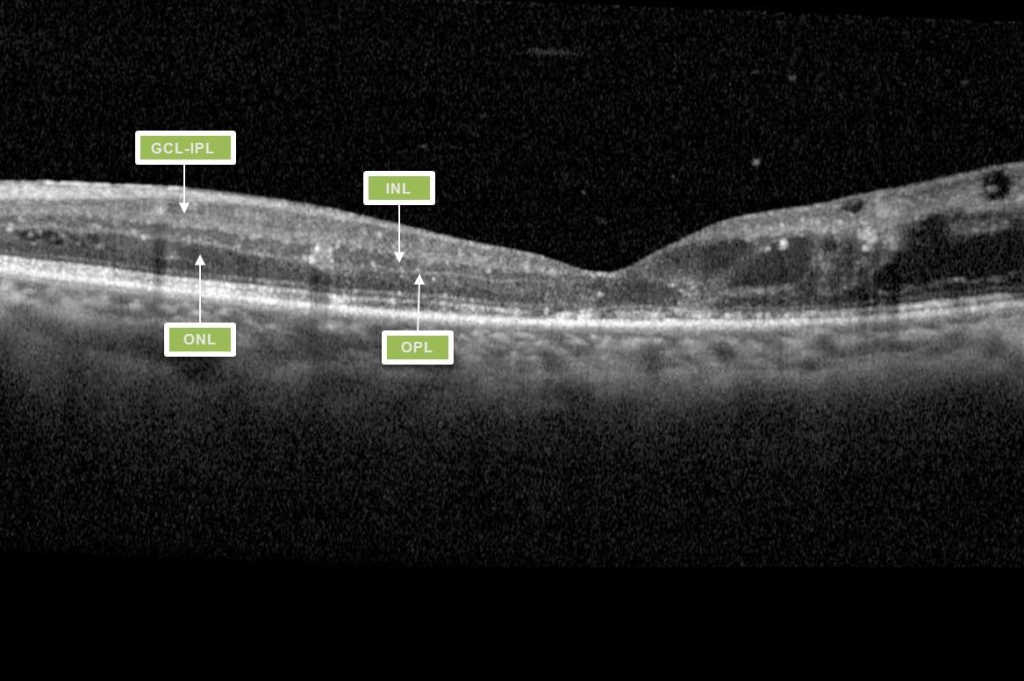
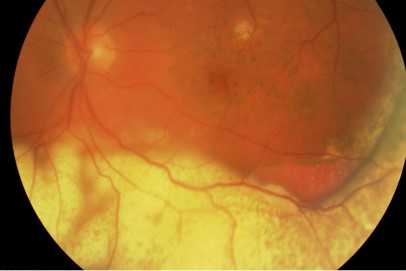
Greater understanding of the ways in which diabetes affects the structure and function of the macula has afforded clinicians a greater predictive measure for assessing a patient’s likely response to treatment. This has been demonstrated with imaging features such as disorganisation of retinal inner layers (DRIL, Fig 1), thought to indicate damaged cells in the inner retinal layer, causing disruption to the normal visual pathway from the photoreceptors to the ganglion cells, resulting in impaired retinal function and likely poor response to treatment9.
DRIL is identified on OCT when there is disruption of the demarcating interface lines between the ganglion cell-inner plexiform complex (GCL-IPL), inner nuclear layer (INL), outer plexiform layer (OPL) and outer nuclear layer (ONL)9. DMO associated with vitreomacular interface abnormalities (VMIA) – notably epiretinal membranes and vitreomacular traction – also tends to have poorer visual outcomes.
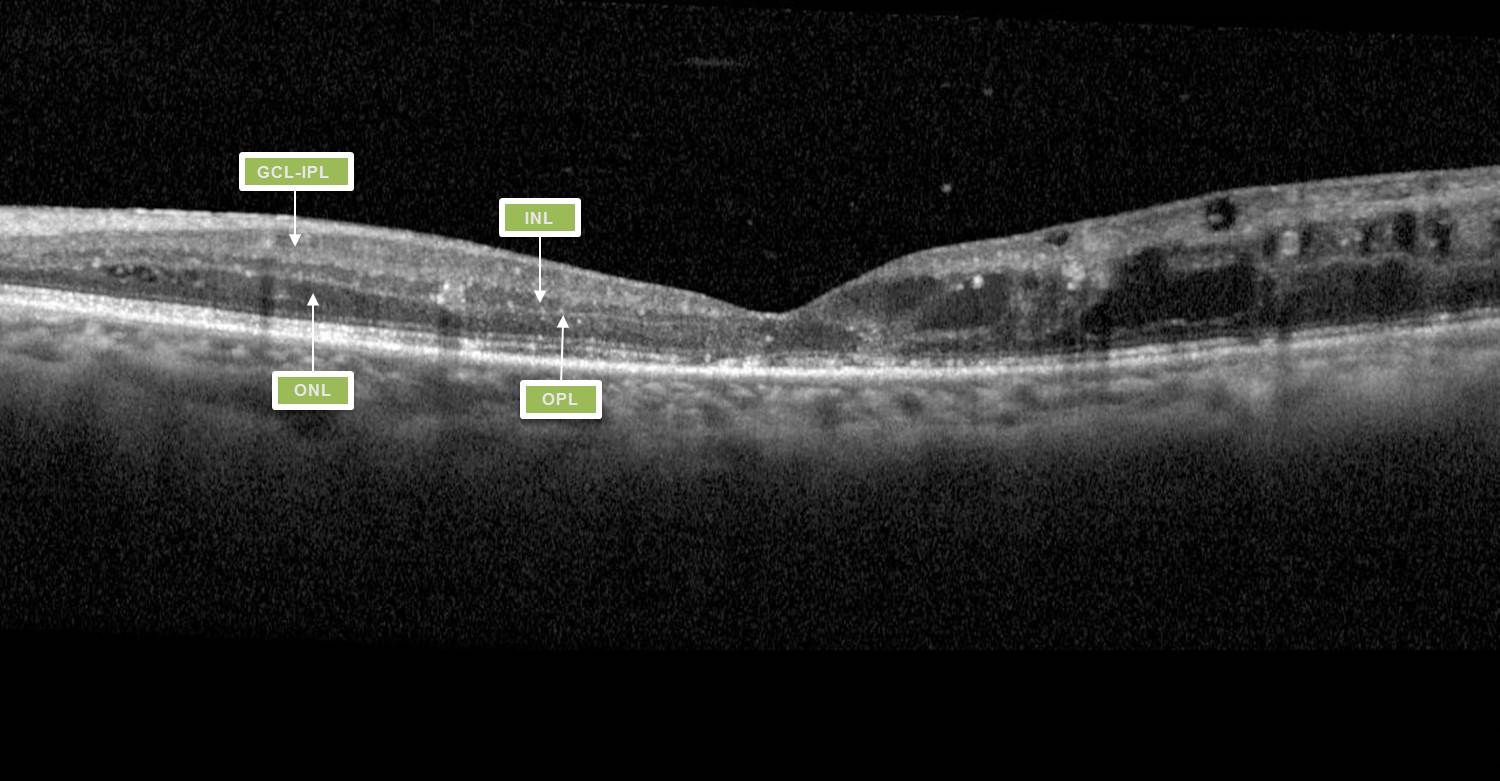
Fig 1. Macula OCT of a patient displaying the features of DRIL. Within the nasal macula the GCL-IPL complex, INL, OPL and ONL can be defined by the hyporeflective and hyperreflective bands. The temporal macula demonstrates loss of this lamination, indicating DRIL.
Another utilisation of OCT is in the differentiation between new vessels elsewhere (NVE) and intraretinal microvascular abnormalities (IRMAs), based on their location in the cross-sectional view on OCT. IRMAs are always contained within the intra-retinal layers whereas neovascularisation occurs along the posterior hyaloid interface (pre-retinal).
More recently, OCT angiography (OCTA) has become clinically available. OCTA demonstrates the perfusion of the macula in the three vascular layers of the retina. A patient whose vision fails to improve with anti-vascular endothelial growth factor (anti-VEGF) treatment despite resolution of their macula oedema, can now be non-invasively imaged with OCTA to look for an enlarged foveal avascular zone (FAZ) indicating macular ischaemia as a reason for the poor response (Fig 2), thus, avoiding the need for a fundus fluorescein angiogram (FFA) investigation for these patients.
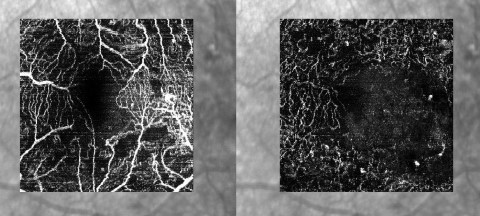
Fig 2. The superficial capillary plexus (SCP) (left) and deep capillary complex (DCP)( right) of a diabetic patient. In the SCP the foveal avascular zone appears irregular and enlarged. More extensive capillary loss is revealed in the DCP image
Widefield OCTA imaging is also available in a few clinical centres. This dye-less imaging modality can demonstrate peripheral vascular perfusion, areas of ischaemia, NVE and new vessels at the disc (NVD). In the future this technology could replace FFA investigations for many patients, except where increased vascular permeability (leakage) is the focus of attention.
Increasing disease burden and more freely available imaging modalities have created an enormous burden of imaging data to assess DR. To meet the clinical demand for the analysis of these images, artificial intelligence (AI) systems have been developed using deep learning algorithms. In the UK, DeepMind's AI system is being used to analyse DR screening images and Google’s Automated Retinal Disease Assessment (ARDA) screening tool is currently in use in India. Within New Zealand, an Auckland-based clinical team has developed an AI platform called Theia to analyse retinal screening images. Trials in Australia, India and Thailand have also used AI to analyse DMO from OCT images.
Virtual medical retina imaging clinics are another approach to tackling the high demand for DR monitoring across the world. Since 2020, Moorfields has run a high-volume virtual medical retinal imaging service, reviewing 1,000 patients every six weeks across its multiple London sites. Such virtual clinics aim to meet the need for the ever-increasing number of patients while reducing face-to-face clinic appointments, especially in this time of Covid-19.
Virtual medical retina clinics have also been in operation for over two years in Auckland. This involves a single appointment where widefield photography and OCT images are taken. A clinician reviews these images and other clinical information in a virtual review a few weeks later. The outcome of the virtual review is communicated via letter to the patient and also copied to their GP. Patients requiring treatment can be identified and booked into face-to-face clinics or directly booked for treatments such as intravitreal injection or retinal laser. While these clinics are able to assess many ocular conditions such as age-related macular degeneration, initial audit data suggests that they are servicing a far greater number of patients with diabetic eye disease than any other condition.
Managing DR
Diabetic macula oedema
Since 2010, anti-VEGF has become the principal treatment of centre-involving (foveal-involving) DMO (ci-DMO). The results of the Diabetic Retinopathy Clinical Research (DRCR) Network trials have made a major contribution to shaping our current clinical practice. For patients with DMO but relatively good vision (6/9.5 to 6/12), Protocol T showed all three anti-VEGF agents (aflibercept, bevacizumab and ranibizumab) give similar visual acuity gains at two years10. However, when a patient’s starting vision is 6/12 or worse, aflibercept treatment resulted in better visual outcomes at two years compared to bevacizumab10. In New Zealand, Pharmac funding for aflibercept is limited to those patients who fail to adequately respond to bevacizumab and who show a better response to a treatment trial of three-monthly aflibercept injections. For patients with ci-DMO and good vision (6/7.5 or better), DRCR Protocol V indicated that observation rather than immediate treatment with anti-VEGF was a reasonable approach, as long as the patient can be sufficiently monitored and treatment initiated if vision worsens11.
Despite the effectiveness of anti-VEGF treatment for macular oedema, DRCR Protocol I showed up to 40% of patients have a suboptimal response to treatment12. A prompt switch to an alternative anti-VEGF agent in these cases can prevent further vision loss. However, real-world data suggests that the cause for an incomplete response is often multifactorial. In many instances insufficient treatment is the most significant factor, which can be influenced by both clinician- and patient-related factors such as clinic capacity, availability of injection appointments and a patient’s ability to attend such frequent appointments.
Proliferative DR
Two recent trials have indicated that anti-VEGF therapy is an effective alternative to panretinal photocoagulation (PRP) for proliferative retinopathy. Both the clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy (Clarity) study and the DRCR Protocol S study showed that eyes treated with anti-VEGF had better or similar visual acuity results to PRP at two years, less visual field loss and less DMO5. However, the follow-up study for Protocol S revealed that the initial gain in visual acuity in the anti-VEGF group is reduced over time, and at five years the visual acuity outcome is similar in both treatment groups5. Worryingly, a high number of patients in the study (30%) had persistent active new vessels in both treatment groups and a third of the patient cohort from the original study had been lost to follow up in the five-year review study5.
Widespread implementation of anti-VEGF for the treatment of PDR comes with significant clinical and financial challenges. Emerging evidence has indicated that anti-VEGF treatment does not improve retinal perfusion, nor prevent progression of non-perfusion. Stopping anti-VEGF therapy can also result in significant visual loss due to rebound neovascularisation. This is particularly pertinent if patients are not monitored regularly. When determining the best treatment option for a patient with PDR the clinician considers the treatment burden and likely compliance with treatment for that individual, as well as the cost and treatment efficacy.
The detection and treatment of DR will continue to represent a significant challenge to the ophthalmic community. Rapidly advancing imaging technology and novel anti-VEGF treatments in the pipeline are our best hope of addressing these challenges.
References
- Sun JK, Aiello LP, Abràmoff M, Antonetti D, Dutta S, Pragnell M, et al. Updating the Staging System for Diabetic Retinal Disease 2021;128:490–3.
- Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. aflibercept, bevacizumab, or ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomised Clinical Trial 2016;123:1351–9.
- Baker C, Glassman A, Beaulieu W, Antoszyk A, Browning D, Chalam K, et al. Effect of Initial Management With aflibercept vs Laser Photocoagulation vs Observation on Vision Loss Among Patients With Diabetic Macular Edema Involving the Center of the Macula and Good Visual Acuity: A Randomised Clinical Trial 2019;321:1880–94.
- Oishi A, Hidaka J, Yoshimura N. Quantification of the image obtained with a wide-field scanning ophthalmoscope 2014;55:2424–31.
- Joltikov K, Sesi C, de Castro V, Davila J, Anand R, Khan S, et al. Disorganisation of Retinal Inner Layers (DRIL) and Neuroretinal Dysfunction in Early Diabetic Retinopathy 2018;59:5481–6.
- International Diabetes Federation. IDF Diabetes Atlas 2021. https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf.
- Jampol L, Glassman A, Sun J. Evaluation and Care of Patients with Diabetic Retinopathy 2020;382:1629–37.
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16-64 years), 1999–2000 with 2009–2010 2014;4:e004015.
- Bressler N, Beaulieu W, Glassman A, Blinder K, Bressler S, Jampol L, et al. Persistent Macular Thickening Following Intravitreous aflibercept, bevacizumab, or ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial 2018;136:257–69.
- Yau J, Rogers S, Kawasaki Y, Lamoureux E, Kowalski J, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy 2012;35:556–64.
- Aiello L, Odia I, Glassman A, Melia M, Jampol L, Bressler N, et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging With Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy 2019;137:65–73.
- Wong Ty, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis 2009;32:2307–13.

Dr Charlotte Jordan is a clinical research fellow in medical retina with the ophthalmology department at the University of Auckland, with clinical supervision by Dr Sophie Hill.

Dr Sophie Hill is a consultant ophthalmologist with specialty training in medical retina based at Auckland District Health Board and Eye Institute.