nAMD research update
Diagnostic accuracy of monitoring tests of fellow eyes in patients with unilateral
nAMD
Sivaprasad S et al
Ophthalmology 128, 1736-1747, December 2021
Review: This is a study of the diagnostic accuracy of patient-reported vision, Amsler grid, visual acuity (VA) measurement in clinic, fundus examination and spectral domain optical coherence tomography (SD-OCT) in detecting conversion to neovascular age related macular degeneration (nAMD) in the fellow eye in patients with nAMD in one eye. The gold standard has traditionally been fundus fluorescein angiography.
In total, 552 patients were recruited and monitored for up to three years. The conversion rate to nAMD in the second eye was 26% over the study period. Apart from OCT, all other tests showed poor sensitivity for detection of conversion to nAMD (Table 1).
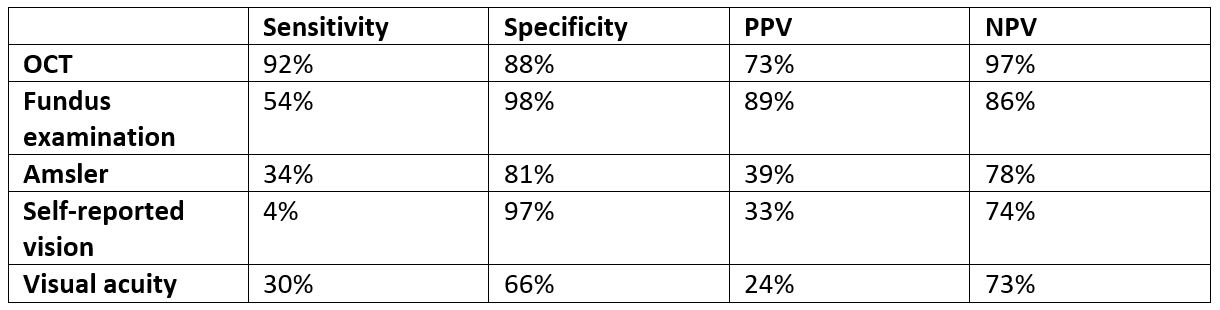
Table 1. Performance of five common monitoring tests in detection of conversion to nAMD in fellow eyes
Comments: The reported conversion rate of 26% over three years is similar to previously published data1. It is of concern that both subjective tests (self-reported vision and Amsler monitoring) and formal VA measurement have poor sensitivity for early detection of nAMD conversion. Sivaprasad et al mentioned that there is a strong emphasis of home-based monitoring for detection of conversion to nAMD using psychophysical technology due to Covid-19. Furthermore, many clinics are avoiding OCT monitoring during the pandemic. Both strategies will increase the risk of under-detection of nAMD conversion in the fellow eye.
In conclusion, it is insufficient to monitor the fellow eye for nAMD based on self-reported symptoms and visual acuity alone. Regular OCT imaging is required for early detection of nAMD and to prevent vision loss.
Outer retinal layer thickening predicts the onset of exudative nAMD
Invernizzi A et al
American Journal of Ophthalmology 231, 19-27, November 2021
Review: Using models based on 3D OCT images and corresponding automatic tissue maps, an artificial intelligence (AI) system is able to predict conversion to nAMD2. However, the specific OCT biomarkers that allow such a prediction are not clearly elucidated.
This study included 47 patients with nAMD in one eye who eventually developed nAMD in the second eye. Serial Spectralis OCT of the fellow eye prior to its conversion to nAMD were analysed.
Comments: This study demonstrates that there is an increase in outer retina thickness in the subfield involved prior to conversion to nAMD, while the outer retina thickness in the uninvolved subfield does not change. There are two detectable ‘break points’ in the thickness/time regression line in the involved sector corresponding to a sudden increase in outer retina thickness, first at 256 days and second at 55 days prior to conversion to nAMD.
As OCT monitoring of the fellow eye of nAMD patients is often opportunistic (usually only at the time of intravitreal treatment), this study provides some clues on predictive biomarkers that indicate imminent conversion to nAMD. Personally, rather than relying solely on the automatic segmentation map, I always have the OCT image of the current visit and the previous visit on comparison mode, and carefully assess the outer retina for any subtle changes in thickness and morphology when looking for nAMD conversion.
Predicting incremental and future visual change in nAMD using deep learning
Fu D J et al
Ophthalmology Retina 2021;5:1074-1084, December 2021
Review: This paper describes a deep-learning segmentation algorithm trained on 137,379 OCT scans from 6,467 eyes of 3,261 patients with nAMD. Predictions on VA were made on 926 eyes.
Interestingly, the VA at 12 months could be predicted reasonably well with a simple model based on VA at baseline and incremental VA changes at the time of induction injection (R2 = 0.656). This model outperforms OCT baseline model alone (R2 = 0.15) or with baseline VA (R2 = 0.378). OCT biomarkers at baseline + VA at baseline + VA and OCT changes after injection only gives marginally better performance (R2 = 0.665) than the simple VA model.
Comments: There is considerable hype regarding the utility of deep learning in ophthalmology. However, this paper demonstrates that a simple predictive model can perform well and more complex models are not always superior.
References
1. Clemons, T. E. et al. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 112, 533–539 (2005).
2. Yim, J. et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 26, 892–899 (2020).

Dr Leo Sheck specialises in medical retina, genetic eye disease, electrodiagnostics for complex retina and optic nerve diseases and cataract surgery, especially with co-existing retinal diseases. He consults for ADHB and Retina Specialists and is chair of the NZ Save Sight Society.