Dry eye and systemic disease
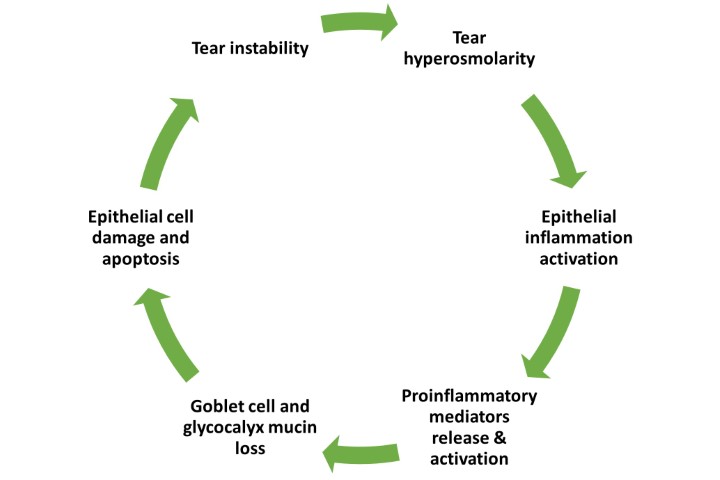
Dry eye disease (DED) is a common condition with a global prevalence estimated at 11.6%1. While DED is often idiopathic, the 2017 Tear Film and Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) highlighted the importance of secondary risk factors associated with systemic diseases2. Systemic diseases can contribute to the development of DED through diverse mechanisms. Neuropathy, for example, as a result of diabetic neuropathy, or because of neurotoxic chemotherapy, can impact ocular surface innervation; autoimmune disorders such as Sjögren syndrome, lupus erythematosus, rheumatoid arthritis, systemic sclerosis and scleroderma, can target the lacrimal glands; and metabolic diseases, such as diabetes mellitus, thyroid disease and hyperlipidaemia, can have a multifaceted impact on the ocular surface. Additionally, other systemic conditions, including cardiovascular diseases, lymphoma, sarcoidosis, graft-versus-host disease, irritable bowel syndrome (IBS), Crohn’s disease and rosacea, have also been linked to DED3,4. Here, we focus on its neuropathic, autoimmune and metabolic risk factors.
Neuropathy
Diabetes can result in diabetic neuropathy in approximately 50% of patients5. This can mean loss of peripheral nerves, and therefore sensation, in the hands and the feet. In extreme cases it can result in ulcers of the feet and even amputation. In the ocular surface, this can lead to a reduction in both the length and number of corneal nerves6. This corneal nerve loss can precede peripheral nerve loss and contribute to a decrease in tear secretion7. In fact, individuals with diabetic neuropathy are more likely to experience DED compared to those without neuropathy8.
DED can also be associated with neurotoxic anti-cancer drug treatments such as paclitaxel (Taxol)9. Mechanistically, this is thought to be due to a reduction in corneal nerve parameters and the inclusion of other drugs such as doxorubicin (Adriamycin), cyclophosphamide, or carboplatin (Paraplatin), commonly included in the treatment regime9. It is important for eyecare clinicians to be mindful of the ocular surface discomfort experienced by cancer patients undergoing neurotoxic chemotherapy. DED symptoms can be experienced even up to a year after treatment cessation9. These medications need to be considered in addition to the previously known iatrogenic causes of DED, such as antihistamines, antidepressants, anxiolytics, isotretinoin (Accutane), anticholinergics, diuretics, beta-blockers and oral contraceptives10.
Autoimmune disease
Autoimmune conditions are characterised by an aberrant immune response that targets the body's own tissues, including the lacrimal glands. Chronic inflammation in these conditions can affect the ocular surface and disrupt the integrity of the tear film, causing DED.
Sjögren syndrome predominantly manifests as aqueous-deficient dry eye, primarily affecting the exocrine glands and leading to diminished tear and saliva production, resulting in DED symptoms11. Similarly, in patients with lupus erythematosus, DED is caused by mechanisms centred on the lacrimal gland, leading to inadequate tear production12. Rheumatoid arthritis mainly affects the joints, but in some cases individuals with this condition may experience DED which could be a result of secondary Sjögren syndrome13. These mechanisms differ from the primary, or idiopathic, cause of DED in the general population, which is mainly characterised by lipid layer disruption and increased tear evaporation14. In patients with thyroid eye disease, incomplete blinking and loss of meibomian gland structure in the upper eyelid is more prominent than in individuals with non-thyroidal dry eye15.
Metabolic disease
Diabetes can lead to DED through various mechanisms other than neuropathy. These mechanisms include decreased tear secretion from microvascular damage to the lacrimal gland caused by hyperglycaemia; reduced binding sites for androgens and oestrogens on the lacrimal gland; damage to the corneal nerves and reduced reflex tearing due to impaired corneal sensitivity; decreased production of mucin by goblet cells, which can reduce tear film stability; and impairment of the meibomian glands16.
A thorough history matters
Given our growing understanding of how systemic disease can impact the ocular surface, it is important that all patients presenting to our practices are questioned regarding both their ocular and medical history. Alarm bells should ring when patients present with systemic conditions linked to DED, such as those discussed here. These patients should be carefully evaluated and managed for their DED and, where possible, this should be reported to both the patient’s GP and their specialist.
Multidisciplinary collaboration in the diagnosis and management of systemic diseases is highly advantageous for patients. Effective management of systemic conditions can help in controlling the progression of DED. As eyecare clinicians, we have the opportunity not only to educate our patients, but to also be part of their holistic care. This collaborative approach significantly minimises delays in diagnosis and ensures timely intervention, thereby improving patient outcomes, both systemically and for DED.
References
1. Papas E. The global prevalence of dry eye disease: a Bayesian view. OPO 2021; 41: 1254-1266.
2. Stapleton F, Alves M, Bunya V et al. TFOS DEWS II epidemiology report. Ocul Surf 2017; 15: 334-365.
3. Henrich C, Ramulu P, Akpek E. Association of dry eye and inflammatory systemic diseases in a tertiary care-based sample. Cornea 2014; 33: 819-825.
4. Kawashima M. Systemic health and dry eye. IOVS 2018; 59: DES138-DES142.
5. Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care 1978; 1: 252-263.
6. Tummanapalli S, Issar T, Kwai N et al. A comparative study on the diagnostic utility of corneal confocal microscopy and tear neuromediator levels in diabetic peripheral neuropathy. Curr Eye Res 2020; 45: 921-930.
7. Misra S, Craig J, Patel D et al. In vivo confocal microscopy of corneal nerves: an ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. IOVS 2015; 56: 5060-5065.
8. Achtsidis V, Eleftheriadou I, Kozanidou E et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care 2014; 37: e210-e211.
9. Chiang J, Goldstein D, Trinh T et al. A cross-sectional study of ocular surface discomfort and corneal nerve dysfunction after paclitaxel treatment for cancer. Sci Rep 2021; 11: 1786.
10. Gomes J, Azar D, Baudouin C et al. TFOS DEWS II iatrogenic report. Ocul Surf 2017; 15: 511-538.
11. Akpek E, Bunya V, Saldanha IJ. Sjögren syndrome: more than just dry eye. Cornea 2019; 38: 658-661.
12. Tseng C-H, Tai Y-H, Hong C-T et al. Systemic lupus erythematosus and risk of dry eye disease and corneal surface damage: a population-based cohort study. Int J Environ Res Public Health 2023; 20: 3776.
13. Abd-Allah N, Hassan A, Omar G et al. Dry eye in rheumatoid arthritis: relation to disease activity. Immunol Med 2020; 43: 92-97.
14. Hakim F, Farooq A. Dry eye disease: an update in 2022. JAMA 2022; 327: 478-479.
15. Park J, Baek S. Dry eye syndrome in thyroid eye disease patients: The role of increased incomplete blinking and Meibomian gland loss. Acta Ophthalmol 2019; 97: e800-e806.
16. Markoulli M, Flanagan J, Tummanapalli S et al. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf 2018; 16: 45-57.

Dr Zahra Tajbakhsh is a PhD candidate and research assistant at the School of Optometry and Vision Science at the University of New South Wales (UNSW).

Associate Professor Maria Markoulli is director of learning and teaching and academic lead of the UNSW Dry Eye clinic at the School of Optometry and Vision Science, UNSW, and a TFOS ambassador.