Alcon unveils Unity
Developed over 10 years, with input from more than 200 surgeons from 30 countries including New Zealand and Australia, the first technologies in Alcon’s new Unity surgical portfolio were introduced to Australasian surgeons at RANZCO 2024.
Work began developing the new portfolio the year after Alcon introduced its current Constellation system, for vitreoretinal and combined procedures, and Centurion for cataract surgery, said Alcon’s international product director, Phaco Equipment, Daniel Velasco. Travelling from Barcelona to Adelaide for the launch, he said: “As we launch one generation, we start working on the next one.”
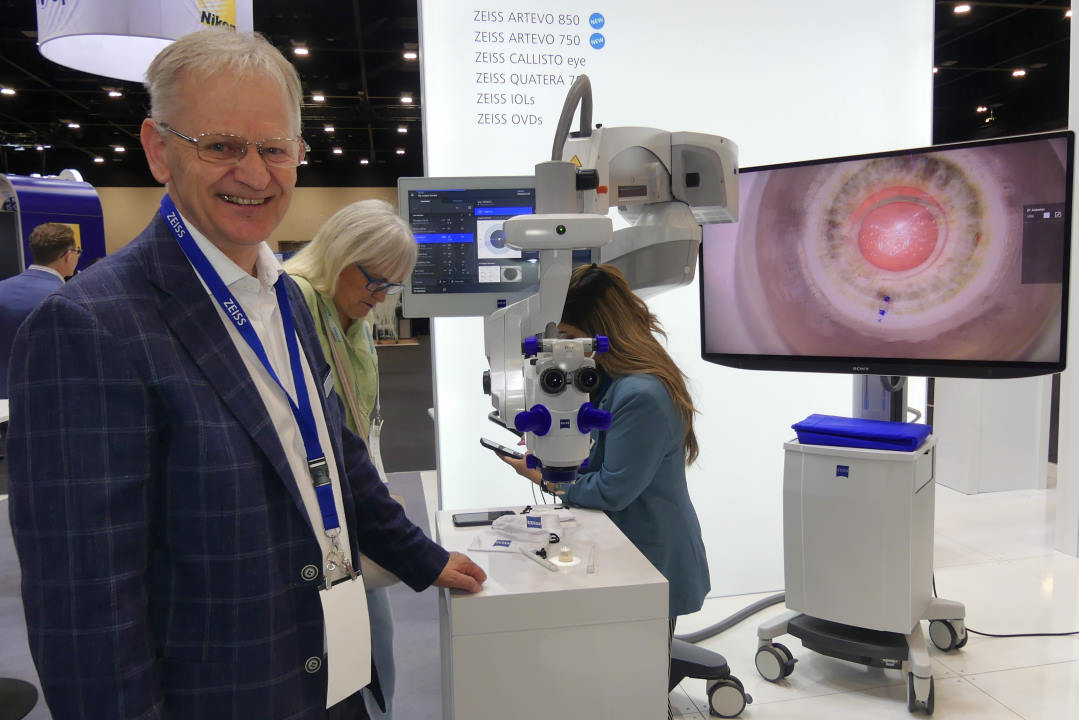
Alcon’s country manager and head of surgical ANZ Penny Stewart said the new Unity vitreoretinal cataract system (VCS) and Unity cataract system (CS) aren’t just polished-up versions of Constellation and Centurion, they are whole new systems with innovative and patented technologies designed to improve efficiencies. Unity VCS and Unity CS leverage a novel phacoemulsification modality allowing nucleus removal to be up to twice as fast as with previous systems, using 40% less energy in the eye, while the vitreoretinal system also offers speeds of up to 30,000 cuts per minute. But, as Stewart explained, it’s not just about improving speeds, it’s also about improving stability, which is provided by a unique proprietary fluidics system, providing a stable, faster system, using less energy, so it’s also more comfortable for the patient.
Though development started a decade ago, most of the testing occurred over the past four years, said Velasco. “We have partnered with some of the top researchers, scientists and high-level surgeons across the world to get their insights and feedback on the technology, but we cannot share names at this point.”
Unity VCS and CS gained US Food & Drug Administration (FDA) approval in June, with Australia and New Zealand the next countries to achieve certification. European approval is expected in early 2025, with the physical rollout of VCS expected mid-2025 and CS in early 2026, said Stewart.
Following the announcement of the systems’ FDA clearance, Tennessee-based vitreoretinal specialist Professor Steve Charles said he’d been closely involved in development. “This truly innovative system is a significant upgrade of Alcon’s best-in-class technologies. It is a proud moment to be able to celebrate this clearance.”
The VCS and CS are the first systems in the new Unity portfolio to be launched, with more expected over the next few years, said Stewart. “We're really excited to have the VCS and CS here… and we’ve been blown away that everything that we think they are, we’re hearing from our surgeons and more. It's certainly a really exciting time for them, because this is going to change the way they operate in the future.”