Bionic eye success in Australia
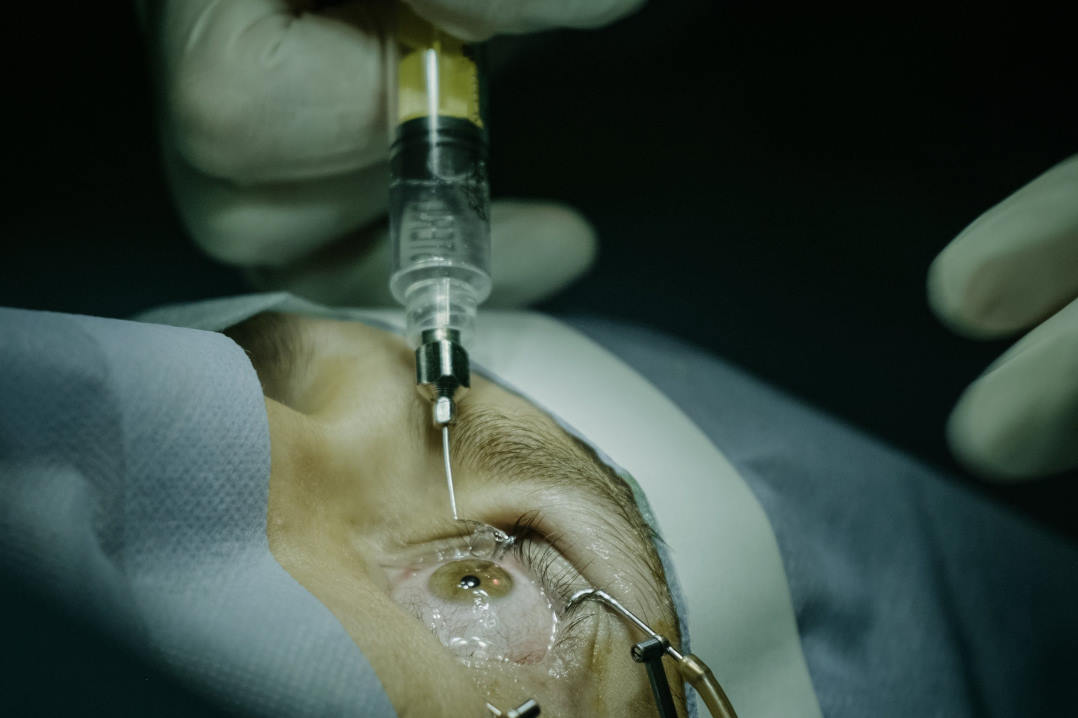
A bionic eye provided ‘substantial improvement’ in the functional vision and quality of life of four patients with retinitis pigmentosa, reported researchers who conducted Australia’s first clinical trial of the device.
Australian company Bionic Vision Technologies’ second-generation bionic eye comprises an electrode, implanted behind the patient’s eye, which receives signals from a video camera mounted on glasses. “The camera converts images into electrical pulses delivered by the electrode array that activate retinal cells and create flashes of light called phosphenes to help patients detect edges, shapes and movement,” said Professor James Fallon, head of research at the Centre for Eye Research Australia (CERA) Bionics Institute, which led the research, together with the University of Melbourne and the Royal Victorian Eye and Ear Hospital.
Over the trial’s two-and-a-half years, patients showed significant improvement in their navigation, mobility and ability to detect objects, said principal investigator Associate Professor Penny Allen. “They reported greater confidence in navigation, were more likely to explore new environments and had reduced need for assistance when travelling to the local shops.”
Participants also reported the bionic eye supplemented long-cane and guide-dog use, provided safe navigation around people and obstacles and allowed them to detect waypoints such as trees and lamp posts along navigational routes, she said. “Patients were able to locate their spouse in a café and detect people moving at a train station – things they could not do (before).”
Published in Ophthalmology Science, the trial’s findings showed the device stayed in place without complications and still had 97% of its electrodes functioning 2.7 years after implantation. The research team is now developing a third iteration of the bionic eye for a worldwide pivotal trial to gain regulatory approval.