Comparing two tear collection methods to characterise inflammatory biomarkers in DED
Activation and elevation of inflammatory mediators is associated with the pathogenesis and progression of dry eye disease (DED)¹. The quantification of inflammatory marker levels can provide insight into the disease progression and severity and may help guide individualised treatment. However, a variety of different tear sampling methods have been described in the literature and their efficacy in quantifying inflammatory markers is not completely understood. Thus, we decided to test the ability of two different collection techniques – the Schirmer strip method and the flush method – to quantify specific inflammatory markers.
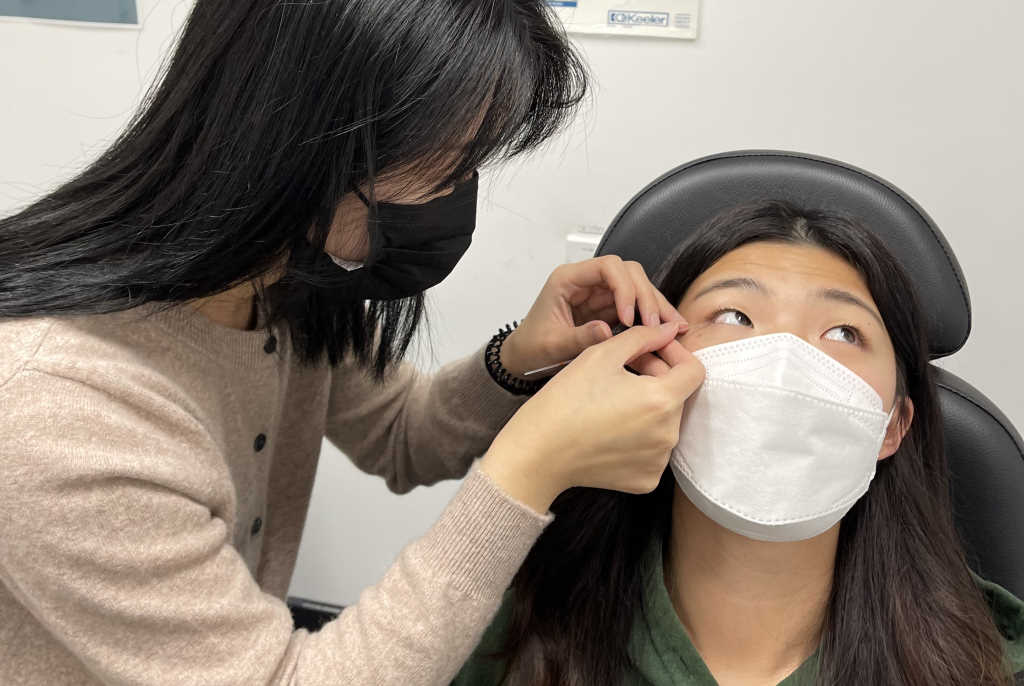
The Schirmer strip test is commonly used to assess lacrimal gland function and identify patients with aqueous-deficient dry eye² and is also regularly employed as a tear sampling method for subsequent laboratory analysis. Similar to the clinical test, sterile absorbent filter paper strips are hooked over the lower lid for a specified amount of time and the tear film is absorbed by the strip³. The strips are then placed in a perforated Eppendorf tube and centrifuged at 10,000-12,000rpm for five minutes to recover the tears³.
The flush method is an efficient method of tear fluid collection that has been described in recent years. A small volume of saline is added to a patient’s ocular surface tears and the resultant fluid is collected for analysis through a microcapillary tube⁴. It is of particular interest to researchers, due to the relative ease of obtaining adequate tear volumes for lab analysis, with minimal reflex tearing and a lower impact on the ocular surface tissues than occurs with the more traditional Schirmer method. This can be helpful when attempting to source basal tears, which represent the basic functional tears of the unstimulated ocular surface. Reflex tearing needs to be avoided in such circumstances as it changes the composition of the collected tear fluid⁵. The flush method has also been proven to preserve proteins and lipid concentrations in extracted tears and works even for patients with low tear volume, for example, those with Sjögren’s syndrome⁶.
During the summer, I co-supervised Part IV optometry summer student Carmen Liu, where we evaluated tear samples collected by the Schirmer strip test and by the flush method. Tears of nine dry eye and nine non-dry eye participants were collected under various conditions: 1) Schirmer strip, single eye sample; 2) Schirmer strip, both eyes pooled; 3) flush method, single eye with 30µL saline; 4) flush method, single eye with 60µL saline; and 5) flush method, both eyes pooled, with 30µL of saline in each. A Luminex High Performance Multiplex Assay (R&D Systems, USA) was used to analyse a range of tear inflammatory markers including IL-1β, IL-6, IL-8, IL10, IL-18, IFN-γ‑ and MMP9. Biomarker profiles across the different test methods were statistically compared using one-way ANOVA and Tukey’s post-hoc test.
The single eye Schirmer strip method was found to yield higher concentrations of IL-18 whereas the single eye flush method with 60µL of saline was superior for MMP-9 and IL-8 quantification. Concentrations of IL-1β, IL-10 and IFN-γ fell below the detection thresholds in the Luminex analysis, possibly on account of intrinsically low inflammatory levels in the recruited study population.
Superiority of one technique over the other was not shown in this study, but rather it was evident that the two studied methods preferentially detect different inflammatory markers, mostly likely due to their relative invasiveness in application, which alters tear composition. What was clear, is the need for researchers to apply consistent methodology for all measurements, as the outcomes from different methods cannot be considered interchangeable. This would be critical, for example, to reliably demonstrate relative change between different disease groups or for monitoring levels of inflammation over time. Further research with a larger sample size and assessing more severe disease is also ongoing.
References
- Ganesalingam K, Ismail S, Sherwin T, Craig JP. Molecular evidence for the role of inflammation in dry eye disease. Clin Exp Optom 2019;102:446–54
- Willems B, Tong L, Dang T, Minh T, Pham ND, Nguyen XH, et al. Novel Cytokine Multiplex Assay for Tear Fluid Analysis in Sjogren’s Syndrome. Ocul Immunol Inflamm 2020:1–6
- Posa A, Bräuer L, Schicht M, Garreis F, Beileke S, Paulsen F. Schirmer strip vs. capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann Anat 2013;195:137–42
- B jerrum KB, Prause JU. Collection and concentration of tear proteins studied by SDS gel electrophoresis Presentation of a new method with special reference to dry eye patients. Graefe’s Arch Clin Exp Ophthalmol 1994;232:402–5
- Sack RA, Kah Ooi Tan, Tan A. Diurnal tear cycle: Evidence for a nocturnal inflammatory constitutive tear fluid. Investig Ophthalmol Vis Sci 1992;33:626–40
- Kuo MT, Fang PC, Chao TL, Chen A, Lai YH, Huang YT, et al. Tear proteomics approach to monitoring Sjögren syndrome or dry eye disease. Int J Mol Sci 2019;20

Dian Zhuang is a PhD student in the University of Auckland’s Ocular Surface Laboratory (OSL), working under the supervision of Professor Jennifer Craig and Dr Stuti Misra. Her research on inflammasomes in dry eye disease is supported by funding from the HRC as part of a collaborative project between OSL and BOTU, led by Associate Professor Ilva Rupenthal. Study collaborators include Catherine Shon, an OSL Masters student, and BOptom honours student Carmen Liu.