GA and AI: a new age
Geographic atrophy (GA) is the late manifestation of non-neovascular age-related macular degeneration (AMD) characterised by progressive loss of retinal pigment epithelium (RPE) and photoreceptors. Until now, it was a slowly progressive untreatable disease that led to vision loss. Emergent trials into the use of complement inhibitors to slow GA progression have shown promising results. Alongside these developments, artificial intelligence (AI) demonstrates efficient and accurate quantitative measurements for disease prognostication and treatment response.
Novel GA treatment
Dysregulation of the complement system is strongly linked to the development and progression of AMD, with evidence coming both from genome-wide association studies and individual case series with rare complement factor H (CFH) gene variants1. Filly is a phase 2 randomised controlled trial evaluating safety and efficacy of pegcetacoplan, a complement C3 inhibitor, for the treatment of GA2. A total of 246 GA patients were randomly assigned to receive 15mg pegcetacoplan intravitreal injection or sham therapy every month or every other month. Eligible patients were at least 50 years of age, had best-corrected visual acuity (BCVA) of 6/95 or better and GA area of 2.5mm2-17.5 mm2. Patients with current or previous neovascular AMD were excluded.
In patients receiving pegcetacoplan monthly or every other month, the GA growth rate was reduced by 29% and 20%, respectively, compared with the sham treatment group. The effect was more pronounced in the second six months of treatment, with observed reductions of 45% and 33% for pegcetacoplan monthly and every other month, respectively (Fig 1). At 12 months, somewhat disappointingly, pegcetacoplan had no effect on changes in foveal encroachment or visual acuity (VA) measures compared with sham treatment. However, direction and rate of GA growth has been shown to be highly variable between individual patients and even at the level of an individual GA lesion3. Local progression is of particular importance, as affection towards the fovea is largely responsible for central vision loss. In-depth assessment of GA is essential to identify patients most likely to benefit from pegcetacoplan, but this is time-consuming (at Moorfields Eye Hospital reading centre, the median time for grading a stack of OCT scans for GA is 43 minutes for a 49-slice volume) and may not be feasible in routine clinical practice.
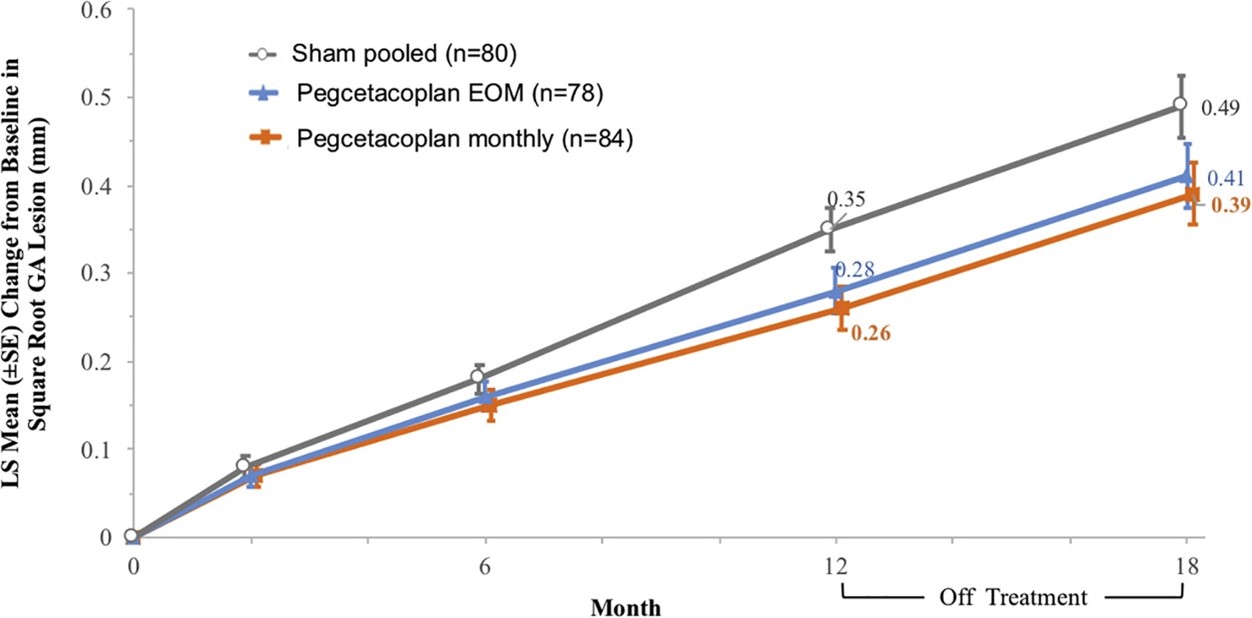
Fig 1. Graph from the FILLY study showing the change from baseline in square root GA
area measurements in the study eye. Credit: Dr David Liao et al and Apellis Pharmaceuticals
AI for patient selection
A group from Moorfields set out to develop and validate a fully automated deep-learning model to detect and quantify GA from optical coherence tomography (OCT)4. The training data was composed of OCT images from the Filly study. The model was then validated on a separate dataset of patients receiving routine care at Moorfields Eye Hospital. The primary outcome was segmentation and classification agreement between the deep-learning model and combined opinion of two expert graders.
When applied to the validation cohort of 884 B-scans from 192 OCT volumes, the deep-learning model produced predictions similar to expert graders’ (median Dice similarity coefficient (DCC) 0.96; intraclass correlation coefficient (ICC) 0.93) and outperformed agreement between human graders (DSC 0.80 [0.28]; ICC 0.79). Similarly, deep-learning models were able to accurately segment each of the three constituent features of GA: retinal pigment epithelium loss (median DSC 0.95), photoreceptor degeneration (0.96), and hypertransmission (0.97) in the external validation dataset, versus consensus grading. In comparison to human grading, it takes the AI model 2.04 seconds to grade a 49-volume OCT stack – a more than 1,000-fold improvement in efficiency.
While treatment response in the Filly trial was based on fundus autofluorescence images, Vogl et al applied a deep-learning model on OCT images from the same dataset5. AI-based analysis automatically segmented the OCT images for pathomorphological features to produce a precise topographic ‘heat map’ of GA activity around existing lesions. Local progression rate was higher for areas with low eccentricity to the fovea, thinner photoreceptor layer, or higher hyperreflective foci concentration in the junctional zone. Even after accounting for these confounding risk factors, there was a statistically significant lowering in the local progression rate by 28% in monthly pegcetacoplan-treated eyes compared with sham.
These two studies demonstrate the capabilities of well-trained AI models in identifying OCT characteristics of GA predictive of visually significant progression. These models achieve an accuracy close to expert human graders, with consistency surpassing interhuman variability. Manual assessment of OCT scans at this level requires expert graders and is time consuming due to intergrader variability. These factors make assessing such endpoints in routine clinical practice or research impractical. AI is the ideal clinical tool to determine which patients will benefit from novel GA treatments and quantify their treatment response for providers, researchers and patients.
Integration of AI into clinical practice
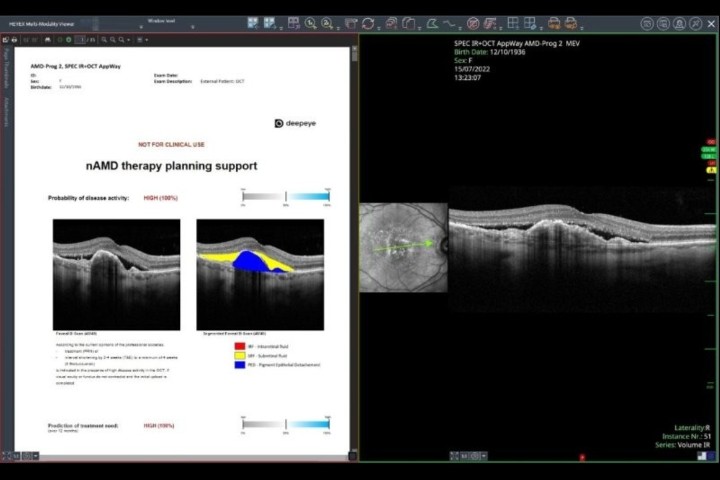
Heidelberg’s AppWay is a new feature offering a secure gateway to transfer images to third-party apps, including AI software, for analysis6. This gateway allows seamless integration of AI algorithms natively into the Heidelberg platform. To date, accessible apps include the RetInSight Fluid Monitor and RetinAI’s apps. The authors anticipate more AI-based algorithms, such as those described above, will be available on this platform to provide clinicians with further insights to optimise patient care.
Editor’s note: for more, see www.nzoptics.co.nz/articles/archive/call-for-ai-to-combat-dr-backlog-in-nz
References
1. Taylor R, Poulter J, Downes S, McKibbin M, Khan K, Inglehearn C, et al. Loss-of-Function Mutations in the CFH Gene Affecting Alternatively Encoded Factor H-like 1 Protein Cause Dominant Early-Onset Macular Drusen. Ophthalmology. 2019;126(10):1410-21.
2. Liao D, Grossi F, El Mehdi D, Gerber M, Brown D, Heier J, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology. 2020;127(2):186-95.
3. Fleckenstein M, Mitchell P, Freund K, Sadda S, Holz F, Brittain C, et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125(3):369-90.
4. Zhang G, Fu D, Liefers B, Faes L, Glinton S, Wagner S, et al. Clinically relevant deep learning for detection and quantification of geographic atrophy from optical coherence tomography: a model development and external validation study. Lancet Digit Health. 2021;3(10):e665-e75.
5. Vogl W-D, Riedl S, Mai J, Reiter G, Lachinov D, Bogunović H, et al. Predicting Topographic Disease Progression and Treatment Response of Pegcetacoplan in Geographic Atrophy Quantified by Deep Learning. Ophthalmology Retina. 2023;7(1):4-13.
6. Heidelberg Engineering GmbH. Heidelberg AppWay www.heidelbergengineering.com/int/heappway.

Dr Leo Sheck specialises in medical retina, genetic eye disease, electrodiagnostics for complex retina and optic nerve diseases and cataract surgery, especially with co-existing retinal diseases. He consults for Te Whatu Ora Auckland and Retina Specialists and is chair of the NZ Save Sight Society.

Dr Aaron Yap is a research fellow in medical and surgical retina at Auckland University. His current research focus is on artificial intelligence and multimodal retinal imaging.