Inflammasomes and DED
The optimal management of dry eye disease (DED) often seems an enigma. A recently published collaborative review by Ocular Surface Laboratory (OSL) and Buchanan Ocular Therapeutics Unit (BOTU) researchers at the University of Auckland outlined current anti-inflammatory options used in clinical management of the disease, including corticosteroids, cyclosporin A and serum topical drops, oral tetracyclines, omega-3 fatty acids and monoclonal antibodies1. The mechanism of action of these pharmaceuticals and their role in disrupting the vicious circle of DED and attempting to restore ocular surface homeostasis is evaluated within the review. While anti-inflammatory medications show clinical and symptomatic benefits in DED, it’s recognised they often fall short of providing safe, long-term relief. Associated side effects can pose sight-threatening risks and discomfort to patients, thus further research and development is needed to overcome these limitations and optimise the efficacy, tolerability and safety of available therapies.
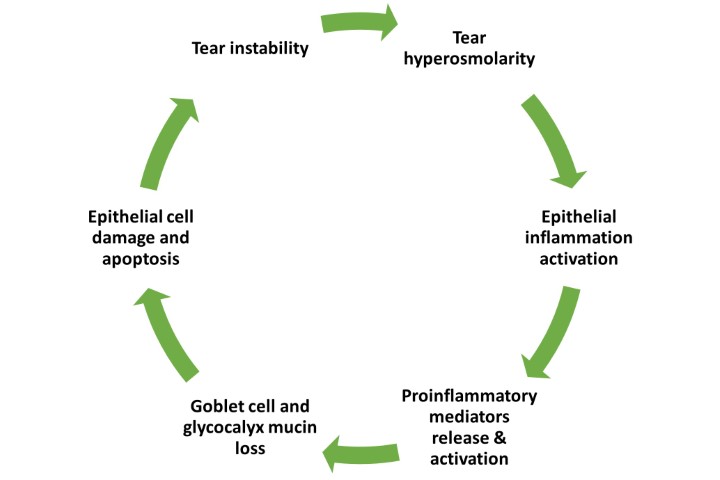
Ongoing research on the inflammasome pathways in ocular surface disease has revealed the potential for a novel, more targeted approach to DED treatment2,3. The NLRP3 inflammasome, a well-studied member of the inflammasome family, plays a crucial role in regulating inflammatory diseases. Growing evidence highlights the importance of NLRP3 in various ocular diseases, including age-related macular degeneration, diabetic retinopathy, Behçet’s disease, uveitis and glaucoma. Over the past decade, the NLRP3 inflammasome has been increasingly implicated in the pathogenesis of DED. Risk factors such as ageing, desiccating environments and contact lens wear contribute to inflammasome activation, leading to the generation of reactive oxygen species and subsequent activation of innate immune responses. This results in elevated production of downstream pro-inflammatory mediators such as interleukin (IL)-1β and IL-18, which drives epithelial and goblet cell loss and glycocalyx disruption – observed clinically as punctate epitheliopathy and tear film instability, triggering and perpetuating the vicious circle of DED, as shown in Fig 1 (above).
Current studies on DED preclinical models are underway to investigate possible therapeutic effects of various anti-inflammatory molecules that target mediators along the NLRP3 inflammasome-mediated signalling pathway. Animal studies have shown topical application of the artemisinin analogue SM934 and mouse adipose-derived mesenchymal stem cell-derived exosomes can suppress the NLRP3 inflammasome pathway and alleviate inflammation in DED models. The reactive oxygen species inhibitor N-acetyl-L-cysteine (NAC) has also been demonstrated to significantly reduce ocular surface signs by downregulating the NLRP3 inflammasome both in vivo and in vitro. Other treatments, such as calcitriol, the active metabolite of vitamin D3; polydatin, a major active component in Polygonum cuspidatum; combined treatment of carboxymethylcellulose, a high-viscosity polymer, and α-melanocyte stimulating hormone, an immunoregulatory neuropeptide; and extracellular vesicles from human adipose-derived stem cells, have shown similar promising results. Additionally, linarin, a natural flavonoid glucoside, has been observed to mitigate corneal epithelial damage by suppressing NLRP3 inflammasome-mediated immunity in a murine DED model, without cytotoxicity. Although the results appear promising, the full extent of the role of the NLRP3 inflammasome activation in DED and its pathophysiological mechanism requires further investigation. Current findings show promise, but further pre-clinical and clinical research is warranted to evaluate the inflammasome inhibitors which might best serve as viable DED therapeutics.
References
1. Zhuang D, Misra S, Mugisho O, Rupenthal I, Craig J. NPRL3 inflammasome as a potential therapeutic target in dry eye disease. Int J Mol Sci. 3AD;24(10866):1-20.
2. Zheng Q, Ren Y, Reinach P, et al. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp Eye Res. 2014;125:1-8.
3. Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS One. 2015;10(5):e0126277.
4. Bron A, de Paiva C, Chauhan S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.

Dian Zhuang is an NZ-trained optometrist and PhD candidate with the OSL and BOTU at the University of Auckland. Dian’s PhD is supported by a Health Research Council grant awarded to BOTU director A/Prof Ilva Rupenthal and her supervisors, Prof Jennifer Craig and Dr Stuti Misra. She gratefully acknowledges a New Zealand Association of Optometrists postgraduate scholarship and the New Zealand Optometric Vision Research Foundation for supporting her PhD studies.