IRD patients welcome gene therapy
In a multinational survey of patients with inherited retinal diseases (IRDs), 90% indicated they were likely or very likely to take up gene therapy if it was available to them.
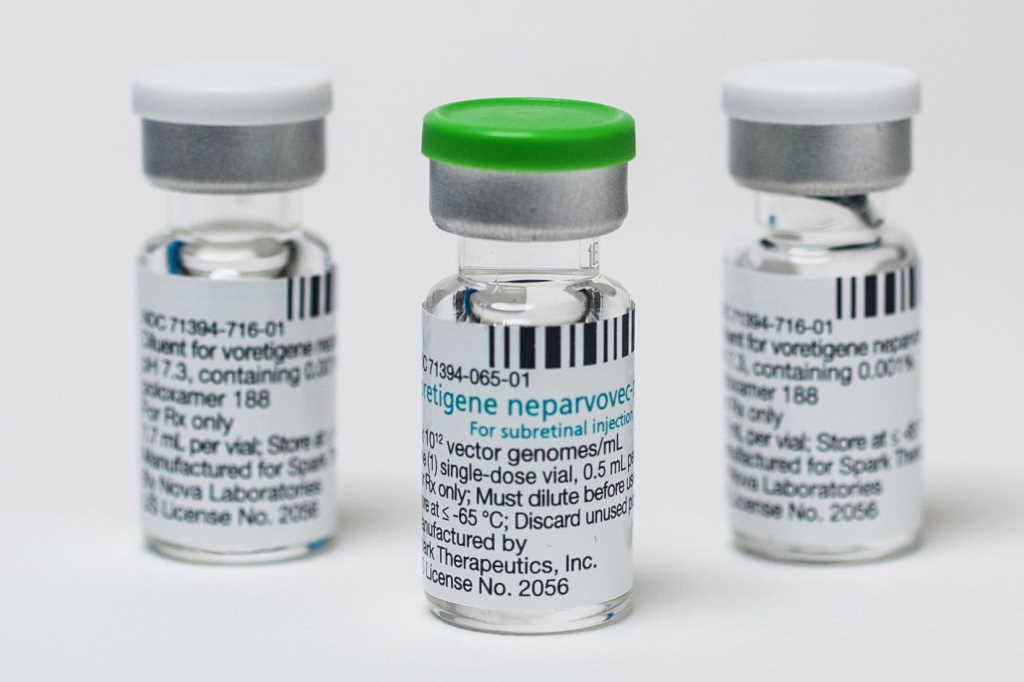
Led by the University of Melbourne’s Dr Ceecee Britten-Jones, the survey respondents reported one or more perceived barriers to receiving gene therapy, with the most common being cost (38%) and fear of side effects (27%). However, almost half (47%) did not know ocular gene therapy is not injected via the arm. Compared to 2021 Australian data, more US-based respondents said they had good knowledge of gene therapy (41% vs 28%), which was reflected in their higher “knowledge of methods” subscale scores, said the research team. Voretigene neparvovec-rzyl (Luxturna) was only approved in Australia in 2021, whereas the first IRD patient treated with ocular gene therapy in the US was in 2007. The US also has more clinical trials and research programmes investigating regenerative IRD treatments, they noted.
Less than 30% of respondents reported receiving information about gene therapy from their ophthalmologist or other healthcare providers, highlighting the importance of clinician education and a need to change management paradigms and advice in light of emerging treatments, said researchers.
The full study was published in Nature.