Landmark AU funding for Luxturna IRD treatment
Luxturna is now funded for patients in Australia with confirmed biallelic RPE65 mutation-associated retinal dystrophy that leads to vision loss and, frequently, complete blindness.
The move follows a recommendation from the Medical Services Advisory Committee and makes Luxturna (voretigene neparvovec), first approved by the Therapeutic Goods Administration in August 2020, the first and only gene therapy to be jointly funded by federal and state/territory governments in Australia, said maker Novartis.
Inherited retinal diseases are linked to more than 260 different genes, including RPE65. A double mutation in RPE65 results in a devastating retinal disease affecting around one in 200,000 people. Just two individuals have been identified with this mutation in the New Zealand Database of Inherited Retinal Diseases, confirmed Associate Professor Andrea Vincent in 2018, when Luxturna received its US Food and Drug Administration approval.
“The funding of Luxturna, the first ocular gene therapy to be available to people with an inherited retinal disease caused by mutations in the RPE65 gene, marks a new era of treatment in Australia,” Dr Tom Edwards, clinical lead for implementation of Luxturna and a vitreoretinal surgeon at The Royal Victorian Eye and Ear Hospital, told MiVision.
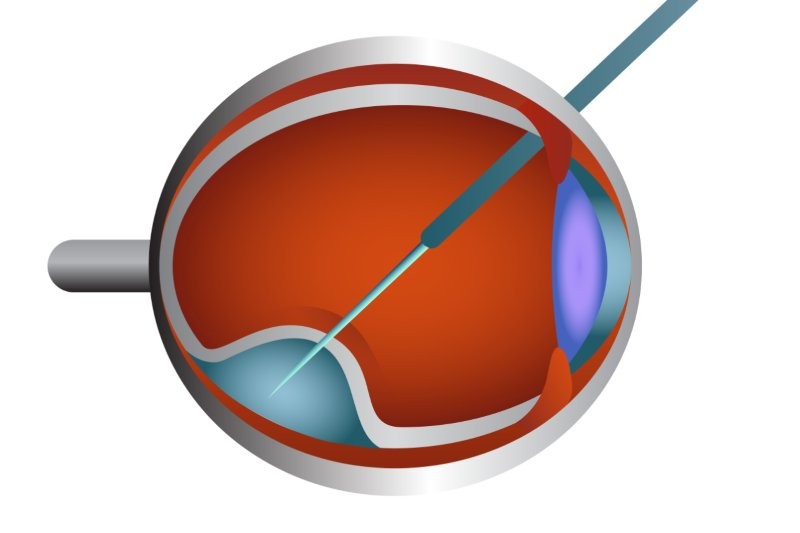
Eligible Luxturna patients must have IRD caused by pathological biallelic RPE65 mutation and have sufficient viable retinal cells, as determined by the treating ophthalmologist. According to Novartis, Luxturna is injected behind the retina and delivers a normal copy of RPE65 directly to retinal cells using an adeno-associated virus vehicle. The therapy is given only once per eye and does not modify any of the patient’s other genes, said the company.