Three-year pegcetacoplan results
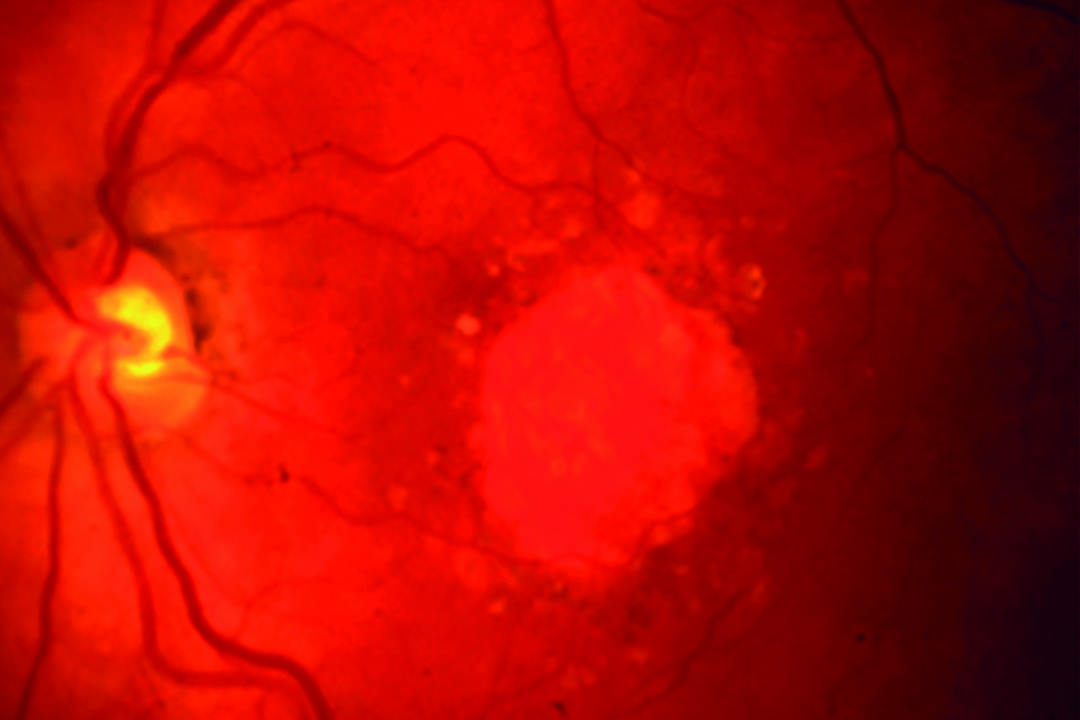
Researchers in Sydney and the US found bi-monthly intravitreal pegcetacoplan (Syfovre) treatment over 36 months showed “increasing efficacy” in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Sydney University’s Associate Professor Samantha Fraser-Bell and colleagues conducted the Gale extension trial, which included 83% of the patients who completed the Oaks and Derby trials that earned pegcetacoplan’s FDA approval in February 2023. Their results showed the mean rate of change in GA area between pegcetacoplan and sham groups increased from 25% in months 0–36 to 35% in year three with monthly injections, and 20% to 24% over the same period for patients receiving injections every other month.
Researchers reported pegcetacoplan’s safety profile was consistent with that of the 24-month Oaks and Derby data. The rate of retinal vasculitis, a previously reported side-effect, was around 1 in 4,000 (0.025%) per first injection, which, they noted, “appears to be a first-injection phenomenon”.