Retina research review – geographic atrophy and diabetic retinopathy
Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration (FILLY trial)
DS Liao et al
Ophthalmology. Feb 2020
Review: This phase 2 randomised clinical trial evaluated the efficacy of pegcetacoplan, a complement C3 inhibitor, for slowing the growth rate of geographic atrophy (GA). Two hundred and forty-six participants were recruited across 46 US sites. Eligible patients were at least 50 years of age, had best-corrected visual acuity (BCVA) of 6/95 or better, confirmed diagnosis of GA secondary to age-related macular degeneration (AMD) using fundus autofluorescence imaging, with an affected area of 2.5-7.5mm2. Studied eyes with a history of retinal disease, including neovascular AMD, were excluded.
Patients were randomised into four groups: 15mg pegcetacoplan monthly, 15mg pegcetacoplan every other month, sham injection monthly, sham injection every other month. At 12 months, participants receiving pegcetacoplan monthly or every other month demonstrated a reduction in GA growth rate by 29% and 20%, respectively, compared with the sham treatment groups. The effect was more pronounced in the second six months of treatment, with observed reductions of 45% and 33% for pegcetacoplan monthly and every other month. Pegcetacoplan had no effect on changes in foveal encroachment, visual acuity measures or low-luminance visual acuity deficit, compared with sham treatment.
There was a higher incidence of endophthalmitis and choroidal neovascularisation (CNV) in study eyes treated with pegcetacoplan. CNV was not associated with any substantial change in visual acuity and those affected were switched on to anti-vascular endothelial growth factor (anti-VEGF) therapy.
Comment: This is the first trial to demonstrate C3 inhibition can slow GA progression. GA progresses slowly and kinetics has been shown to be highly variable between individual patients and even at the level of an individual GA lesion. Patients at highest risk of vision loss from GA likely represent those who would most benefit from current therapy. Further research has been done to subclassify OCT biomarkers that are predictors of GA development with the aid of artificial intelligence image processing algorithms.
Four-year visual outcomes in the Protocol W randomised trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy
RK Maturi et al
JAMA. Feb 2023
Review: The DRCR Retina Network’s Protocol W poses the question, 'Does prophylactic anti-VEGF treatment in eyes at high risk of vision-threatening complications of diabetes result in better anatomic and vision outcomes at four years when compared with observation and treatment only after a vision-threatening complication has occurred?’
Eligible participants were adults with type 1 or type 2 diabetes who had at least one eye with moderate to severe non-proliferative diabetic retinopathy (NPDR) (Diabetic Retinopathy Severity Scale (DRSS) levels 43-53) and best-corrected visual acuity at least 6/7.5 or better. Eyes with current centre-involving diabetic macular oedema (CI-DMO), proliferative diabetic retinopathy (PDR) or evidence of treatment for this in the past 12 months were excluded.
Participants were randomised into two groups: 2.0mg aflibercept (n=200) and sham injections (n=199). Eight injections were administered at defined intervals over two years, then quarterly through to four years unless the eye improved to mild NPDR or better. Aflibercept was commenced if high-risk PDR or CI-DMO with vision loss (two lines at one visit or one line at two consecutive visits) developed.
The four-year cumulative probability of developing PDR or CI-DMO with vision loss was 33.9% with aflibercept vs 56.9% with sham (adjusted hazard ratio, 0.40). As with the PANORAMA study and two-year results of this trial, there was no significant difference in visual acuity from baseline to four years (-2.7 letters with aflibercept vs -2.4 letters with sham). From baseline to four years, DR grading improved to mild NPDR in 50.4% of eyes in the aflibercept group and 29.3% of eyes in the sham group. More eyes in the aflibercept group improved by two or more steps in DRSS (54.3% vs 28.5%; adjusted odds ratio (OR) 2.97) and fewer in the aflibercept group worsened by two or more steps (11.0% vs 22.8%; OR 0.51).
The authors concluded that at four years, treatment of NPDR with aflibercept resulted in statistically significant anatomic improvements, but no improvement in visual acuity. Among four-year completers, the treatment group received a higher number of mean aflibercept treatments compared to sham (13.0 vs 8.7).
Comment: This study demonstrates anti-VEGF use in moderate to severe NPDR is protective against progression to PDR. This is an important consideration when anti-VEGF therapy is ceased; for example, when switching to intravitreal steroid therapy or upon achieving successful resolution of macula oedema. In such cases, fluorescein angiogram is a useful adjunct for quantifying the degree of retinal ischaemia and risk of PDR once the protective effect of anti-VEGF wears off.
The sham group, of which 12% of participants had type 1 diabetes and the mean HbA1c was 8.8%, provides valuable information about the natural history of untreated moderate to severe NPDR. Over four years, approximately 49%, 12% and 19% developed PDR, high risk PDR and CI-DMO, respectively. A third of eyes improved to mild NPDR.
As there was no significant difference in visual acuity between groups, prophylactic treatment with anti-VEGF in patients at risk of developing PDR is not indicated. Pro re nata (PRN) treatment method did just as well in terms of visual acuity outcomes at four years. However, the overall management of diabetes should take into account underlying systemic health, diabetic control and ability to attend regular follow-up appointments.
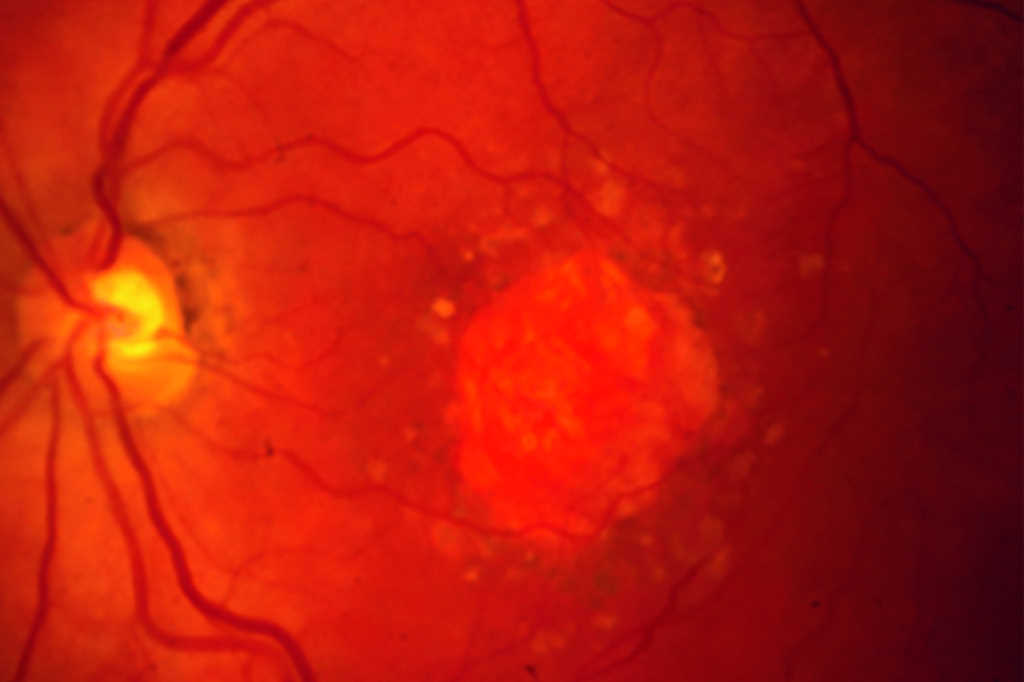
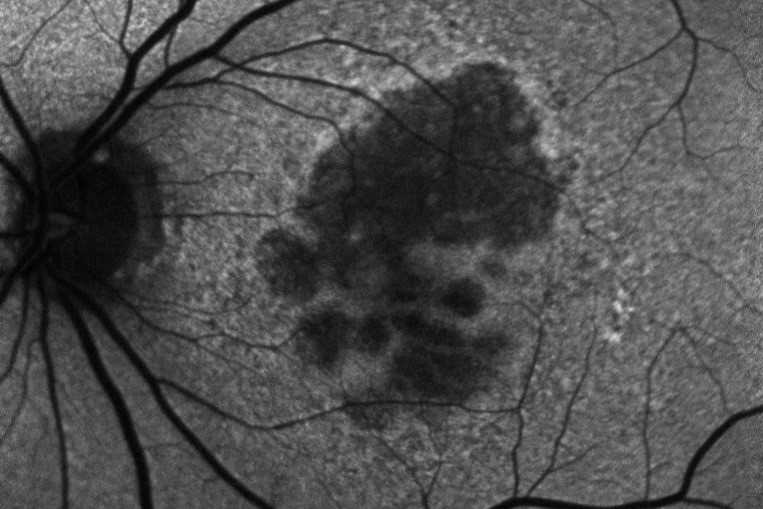
Local anatomic precursors to new-onset geographic atrophy in age-related macular degeneration as defined on OCT
MV Pasricha et al
Ophthalmology Retina. May 2021
Review: This was a retrospective study aimed at characterising the optical coherence tomography (OCT) features of intermediate age-related macular degeneration (AMD) that precede and predict the risk of progression to advanced AMD. The sample population was derived from the Age-Related Eye Disease Study (AREDS 2) dataset, a large prospective database that captured the long-term follow-up of eyes with intermediate AMD.
The study recruited 349 participants aged 50-85 years with at least one eye with large drusen and without central GA or choroidal neovascularisation. OCT features in an area of new-onset GA underwent pair-wise comparison with one size-matched control region in the same eye over the preceding four years. All scans were evaluated by a masked grader.
Investigators were able to demonstrate the following features, in order of appearance, were more prevalent in GA precursor regions compared to control regions: greater drusen volume, choroidal hypertransmission, hyperreflective foci, OCT-reflective drusen substructures, disruption of the photoreceptor layer, ellipsoid zone and retinal pigment epithelium.
The aforementioned OCT features have been shown in multiple studies1-4 to be predictive of GA. While this study did not identify new risk factors, it was able to demonstrate the temporal sequence of these signs. Photoreceptor thinning and higher drusen volume was detected as early as four years prior to GA onset, while hypertransmission and hyperreflective foci came as late signs.
Comment: Specific OCT findings infer a higher risk of progression onto GA. As new treatments for GA are now on the horizon, it is important for ophthalmologists to familiarise themselves with these specific signs to identify those patients who are most likely to benefit. In the future, it is likely that many of these features will be incorporated into an AI-based algorithm to help with clinical decision making.
References
- Jaffe G, Chakravarthy U, Freund K, Guymer R, Holz F, Liakopoulos S, et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration: classification of atrophy meeting report 5. Ophthalmology retina. 2021;5(9):855-67.
- Lei J, Balasubramanian S, Abdelfattah N, Nittala M, Sadda S. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology. 2017;255:1551-8.
- Nassisi M, Lei J, Abdelfattah N, Karamat A, Balasubramanian S, Fan W, et al. OCT risk factors for development of late age-related macular degeneration in the fellow eyes of patients enrolled in the HARBOR study. Ophthalmology. 2019;126(12):1667-74.
- Sadda S, Abdelfattah N, Lei J, Shi Y, Marion K, Morgenthien E, et al. Spectral-domain OCT analysis of risk factors for macular atrophy development in the HARBOR study for neovascular age-related macular degeneration. Ophthalmology. 2020;127(10):1360-70.

Dr Leo Sheck specialises in medical retina, genetic eye disease, electrodiagnostics for complex retina and optic nerve diseases and cataract surgery, especially with co-existing retinal diseases. He consults for Te Whatu Ora Auckland and Retina Specialists and is chair of the NZ Save Sight Society.

Dr Aaron Yap is a research fellow in medical and surgical retina at Auckland University. His current research focus is artificial intelligence and multimodal retinal imaging.